Simulating the Quantum World with Electron Traps
- Details
- Published: Wednesday, August 02 2017 14:30
Quantum behavior plays a crucial role in novel and emergent material properties, such as superconductivity and magnetism. Unfortunately, it is still impossible to calculate the underlying quantum behavior, let alone fully understand it. Scientists of QuTech, the Kavli Institute of Nanoscience in Delft and TNO, in collaboration with ETH Zurich and the University of Maryland, have now succeeded in building an "artificial material" that mimics this type of quantum behavior on a small scale. In doing so, they have laid the foundations for new insights and potential applications. Their work is published today in Nature.
Over the past century, an increased understanding of semiconductor materials has led to many technological improvements, such as computer chips becoming ever faster and smaller. We are, however, gradually reaching the limits of Moore's Law, the trend that predicts a doubling in computing power for half the price every two years. But this prediction ignores the possibility that computers might harness quantum physics.
"There is so much physics left to discover if we truly want to understand materials on the very smallest scale," says Lieven Vandersypen, a professor at TU Delft in the Netherlands and the lead experimentalist on the new paper. And that new physics is set to bring even more new technology with it. "The difficulty is that, at this scale, quantum theory determines the behavior of electrons and it is virtually impossible to calculate this behavior accurately even for just a handful of electrons, using even the most powerful supercomputers," Vandersypen says.
Scientists are now combining the power of the semiconductor industry with their knowledge of quantum technology in order to mimic the behavior of electrons in materials—a technique known as quantum simulation. "I hope that, in the near future, this will enable us to learn so much about materials that we can open some important doors in technology, such as the design of superconductors at room temperature, to make possible loss-free energy transport over long distances, for example," Vandersypen says.
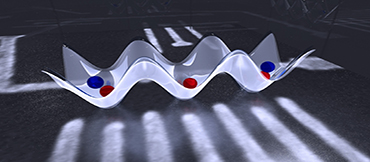 Micrograph image of semiconductor quantum chip with lattice visualization above. By applying voltages on "gates" (white lines), electrons (red and blue spheres) can be captured in quantum dots. The potential landscape (white wave) determines the locations where the electrons are captured. (Credit: Graphic by E. Edwards/JQI, micrograph courtesy of the authors.)
Micrograph image of semiconductor quantum chip with lattice visualization above. By applying voltages on "gates" (white lines), electrons (red and blue spheres) can be captured in quantum dots. The potential landscape (white wave) determines the locations where the electrons are captured. (Credit: Graphic by E. Edwards/JQI, micrograph courtesy of the authors.)
Mimicking nature
It has long been known that individual electrons can be confined to small regions on a chip, known as quantum dots. There are, in principle, suitable for researching the behavior and interactions of electrons in materials. The captured electrons can move, or tunnel, between the quantum dots in a controlled way, while they interact through the repulsion of their negative charges. "Processes like these in quantum dots, cooled to a fraction of a degree above absolute zero, are perfectly suitable for simulating the electronic properties of new materials," says Toivo Hensgens, a graduate student at TU Delft and the lead author of the paper.
In practice, it is a major challenge to control the electrons in quantum dots so precisely that the underlying physics becomes visible. Imperfections in the quantum chips and inefficient methods of controlling the electrons in the dots have made this a particularly hard nut to crack.
Quantum equipment
Researchers have now demonstrated a method that is both effective and can be scaled up to larger numbers of quantum dots. The number of electrons in each quantum dot can be set from 0 to 4 and the chance of tunnelling between neighbouring dots can be varied from negligible to the point at which neighbouring dots actually become one large dot. "We use voltages to distort the (potential) landscape that the electrons sense," explains Hensgens. "That voltage determines the number of electrons in the dots and the relative interactions between them."
In a quantum chip with three quantum dots, the QuTech team has demonstrated that they are capable of simulating a series of material processes experimentally. But the most important result is the method that they have demonstrated. "We are now easily able to add more quantum dots with electrons and control the potential landscape in such a way that we can ultimately simulate very large and interesting quantum processes," Hensgens says.
The Vandersypen team aims to progress towards more quantum dots as soon as possible. To achieve that, he and his colleagues have entered a close collaboration with chipmaker Intel. "Their knowledge and expertise in semiconductor manufacturing combined with our deep understanding of quantum control offers opportunities that are now set to bear fruit," he says.
This story was prepared by the Delft University of Technology (TU Delft) and adapted with permission. The experiments described were performed at TU Delft, with theoretical and numerical contributions from JQI Fellow and Condensed Matter Theory Center Director Sankar Das Sarma and JQI postdoctoral researcher Xiao Li.
Research Contacts:
Professor Lieven Vandersypen
This email address is being protected from spambots. You need JavaScript enabled to view it.
Dr. Xiao Li
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Contact: